43 quality control records
Quality Control Quality Control is a full-service boutique management agency that curates custom brand relationships tailored to specific talent. lat: ° lng: ° ... 11 Food Quality Control Procedures That Every Company Should Know Shipping is the last step in which food businesses have direct control over product quality. Ship items on a FIFO/FEFO basis and use the same guidelines in shipping that were set up in receiving. 10.
Quality Control Music - Wikipedia Quality Control Music (also known as Quality Control or QC) is an American hip hop record label founded by Kevin "Coach K" Lee and Pierre "P" Thomas in March 2013. Its productions were distributed through Universal Music Distribution until it was dismantled in 2015; the label's releases are now distributed through Motown and Caroline, subsidiaries of the Capitol Music Group.

Quality control records
New approach to document and record control in ISO 9001:2015 - 9001Academy ISO 9001:2015 defines documented information as meaningful data that is required to be controlled and maintained by the organization and the medium on which it is contained. Notes to this definition indicate that documented information can refer to the Quality Management System (QMS) and its processes, documentation, and records. Quality Systems - Control of Documents Get a MSWord file now, formatted with required document control information for $2. #2 - Nominate a single place to keep master copies and a register of documents This is where end users will go to check whether the version they have is the latest version. It may also be the place where they access the documents they need. 5 Examples of Quality Control - Simplicable Quality control is the process of detecting mistakes in operational outputs such as products and services. This can involve testing every single output such as the products off an assembly line. Alternatively, it can involve taking statistically significant test samples that provide confidence that results are to specifications.The following are illustrative examples of quality control.
Quality control records. Data Entry on Quality Control Laboratory Records - GMPSOP Notebooks and worksheets are essential for supporting and verifying accuracy, reliability and authenticity of raw data and results. Laboratory records are important for the following reasons: providing documented evidence that tests were actually conducted. allowing checks by a second analyst to be undertaken. Quality control - Wikipedia Quality control ( QC) is a process by which entities review the quality of all factors involved in production. ISO 9000 defines quality control as "A part of quality management focused on fulfilling quality requirements". [1] This approach places emphasis on three aspects (enshrined in standards such as ISO 9001): [2] [3] Quality Control Records Investigator The Quality Records Investigator will be responsible for tracking, management, and completion of Quality Control Quality Records (such as deviations, lab investigations, change control, and CAPA) in partnership with applicable Subject Mater Experts. Press — Quality Control November 19, 2019. Coach K, the titan behind Atlanta's blooming hip hop community. Quality Control, the label behind hip hop icons like Lil Baby, Migos, and City Girls, was co-founded by Kevin "Coach K" Lee, who carries with him a large presence. He has a deep, restrained voice, and a stark white beard.
Policy Guidance Help System - What quality assurance (QA) records must ... 3) Quality Control (QC) test Records: including QC test procedures, test performance and monitoring, data analysis and timely corrective actions for each. 4) Procedures for safety and protection of patients and personnel. Control of Quality Records - EBME Control of Quality Records Details Last Updated: 13 November 2015 Purpose The purpose of this procedure is to ensure that all EBME Quality Records are correctly managed, and that the associated responsibilities are defined. Scope This procedure applies to all quality-relayed records held by the EBME Department. Definitions ISO 9001 QMS documentation - How to structure it - 9001Academy The QMS documentation can consist of different types of documents. Usually, it includes documents such as the Quality Policy, Quality Manual, procedures, work instructions, quality plans, and records. The QMS documentation can be represented as a hierarchy, as shown in the diagram below: ISO 9001 requires different types of information to be ... PDF CHAPTER 13 Quality Control/Quality Assurance - Centers for Disease ... Examples of records include request forms, report forms, logbooks, quality control results, patient reports, critical communications, and notices from hospitals or public health authorities. See Chapter 3: Results Management and Reporting of Data for items that should be included in
Quality policy statement examples: Create your best quality ... - Sitemate A quality policy statement is a short document published by the quality team or management of an organisation to establish what quality means to that organisation. Quality policy statements are always used internally as a means of establishing a quality baseline for all employees to adhere to, while they are commonly made public and available ... Policy Guidance Help System - Quality Assurance Records Quality Assurance Records Citation: 900.12(d)(2): Quality assurance records. The lead interpreting physician, quality control technologist, and medical physicist shall ensure that records concerning mammography technique and procedures, quality control (including monitoring data, problems detected by analysis of that data, corrective actions, and the effectiveness of the corrective actions ... Free Quality Control Templates | Smartsheet While quality assurance (QA) is focused on quality process, quality control is focused on output quality — and you need a comprehensive document to ensure this level of quality control. Use this template to specify your QC policy, so that all production personnel understand and adhere to it. Quality Records - How long do you keep inspection records and why? Integrating acquired existing product into Quality Management System Records: ISO 9000, ISO 9001, and ISO 9004 Quality Management Systems Standards: 2: Jun 5, 2013: A: Are all In-Process Quality Records required in final QA Batch Record Review. 21 CFR Part 820 - US FDA Quality System Regulations (QSR) 9: Sep 10, 2012: K: Quality Records (QR ...
Retention of Quality Control (QC) Records - USDA Retention of Quality Control (QC) Records Home Supplemental Nutrition Assistance Program (SNAP) The purpose of this policy memo is to notify state agencies of the specific record retention requirements for recent QC review periods. As required by regulations, QC records must be retained for three years following fiscal closure.
Quality Records Definition | Law Insider Quality Records means Documents containing recorded information, regardless of the medium or characteristic, which demonstrate the effectiveness of the quality management system and that provide evidence that products meet regulatory requirements and comply with specified product requirements. Sample 1 Sample 2 Sample 3 Based on 5 documents
Roles and Responsibilities of a Quality Control Inspector A "Quality Control Inspector" monitors the quality aspects of incoming raw materials to the organization and the products that are ready to be shipped. They also ensure that manufactured products meet the specified quality standards set by the organization before being sent to customers. The quality control inspector job role involves ...
Quality Control Laboratory Compliance - Documentation and Record ... Records are management tools that help in the continuous management of the quality system. They also help track samples throughout the process and identify problems. They indicate how your staff has been operating. Poor record keeping is often an indication of poor performance and disorganization. Records help in decision making.
Control of Records in ISO 9001:2008 | Keeping Records The ISO 9000 standard has a built-in reference to all required records wherever the phrase "see 4.2.4″ is found (4.2.4 is the paragraph dealing with Control of Records). The 20 mandated ISO9000 records are: Document Control (4.2.3) Management Review (5.6.1) Education, Training, Skills and Experience (6.2.2) Product Realization (7.1)
Control of Records - ISO 9001 Help The following clauses of ISO 9001 contain the instruction 'see 4.2.4' which means that you must retain these 21 records: 5.6.1 Management review minutes 6.2.2 Records of education, training, skills and experience 7.1 Evidence that the realization processes and product fulfil requirements 7.2.2 Records of sales activities
Maintenance | QUALITY CONTROL HQ A home for UKHC, NWOBHC and friends.
Exploring ISO 9000 - Part 16 Control of Quality Records The approach for control of quality records is to identify the records required in each of your procedures to support or provide objective evidence that the procedure has been followed. It is suggested that you develop one procedure to comply with Clause 4.16.
Quality Records - Vintara Quality Management System The quality record must also demonstrate the effective operation of the QMS. For example, a newly created test procedure is not a quality record, although generally it is a controlled document. It becomes a quality record when it is used to record the steps executed during a specific test to establish that the product met requirements.
The Quality Assurance And Quality Control Reports 2. Monthly Quality Report This report will be needed and it shall be incorporated in the Monthly Report of the project that will be submitted to the consultant and client. It's a general quality report of the project. See below the sample quality report. 3. The Internal Monthly Report
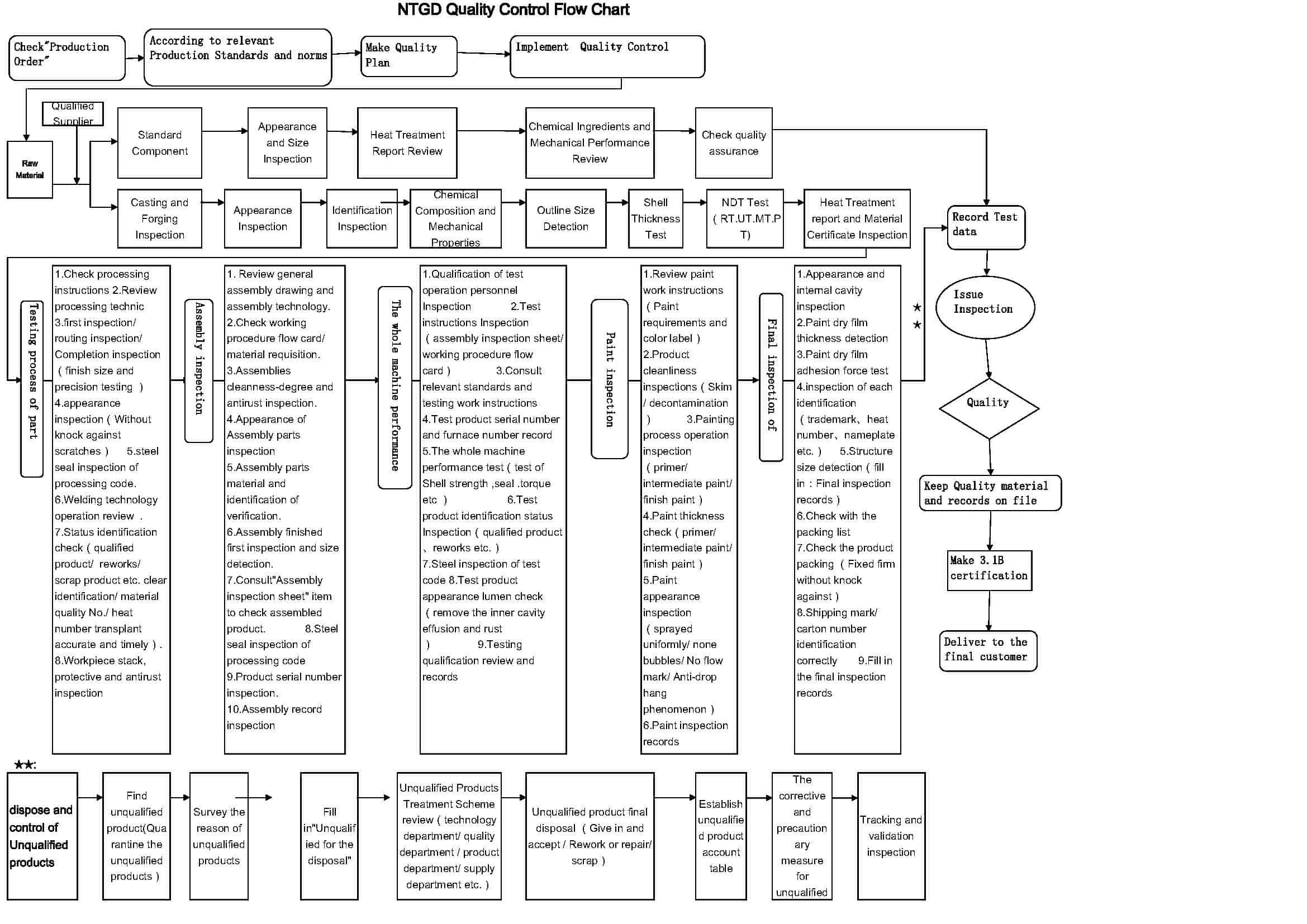
PDF Quality Control Records - Nsh
5 Examples of Quality Control - Simplicable Quality control is the process of detecting mistakes in operational outputs such as products and services. This can involve testing every single output such as the products off an assembly line. Alternatively, it can involve taking statistically significant test samples that provide confidence that results are to specifications.The following are illustrative examples of quality control.
Quality Systems - Control of Documents Get a MSWord file now, formatted with required document control information for $2. #2 - Nominate a single place to keep master copies and a register of documents This is where end users will go to check whether the version they have is the latest version. It may also be the place where they access the documents they need.
New approach to document and record control in ISO 9001:2015 - 9001Academy ISO 9001:2015 defines documented information as meaningful data that is required to be controlled and maintained by the organization and the medium on which it is contained. Notes to this definition indicate that documented information can refer to the Quality Management System (QMS) and its processes, documentation, and records.


































Post a Comment for "43 quality control records"